Worksheet names of ionic compounds provide a structured approach to understanding the nomenclature of these essential chemical entities. This guide delves into the fundamental concepts of ionic compounds, exploring their properties, naming conventions, and practical applications.
By understanding the principles behind worksheet names of ionic compounds, students can develop a strong foundation in chemical nomenclature and enhance their problem-solving abilities.
Ionic Compounds: Worksheet Names Of Ionic Compounds

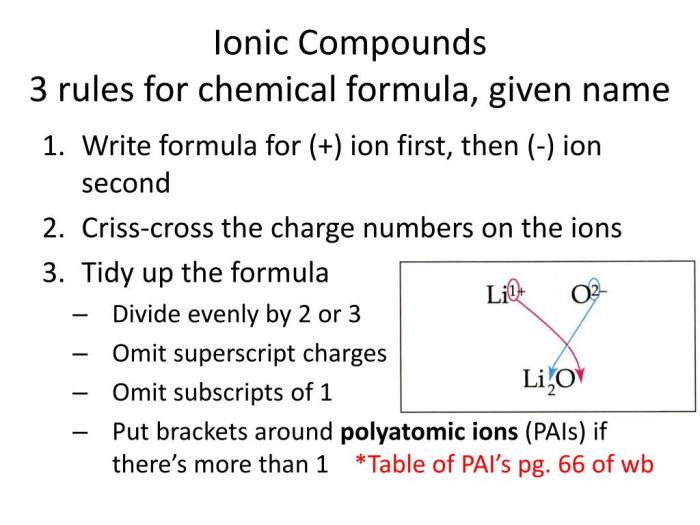
Ionic compounds are formed when a metal loses one or more electrons to a nonmetal. The resulting positively charged ion (cation) is attracted to the negatively charged ion (anion) to form an ionic bond.
Examples of ionic compounds include sodium chloride (NaCl), potassium iodide (KI), and calcium oxide (CaO).
Ionic compounds typically have high melting and boiling points, are soluble in water, and conduct electricity when dissolved in water or melted.
Nomenclature of Ionic Compounds
The rules for naming ionic compounds are as follows:
- The cation is named first, followed by the anion.
- The cation name is the same as the name of the metal.
- The anion name is the root of the nonmetal name followed by the suffix
ide.
For example, the ionic compound NaCl is named sodium chloride. The cation is Na+, which is the sodium ion. The anion is Cl-, which is the chloride ion.
There are some exceptions to the naming rules. For example, the anion of oxygen is oxide, not oxyide. The anion of sulfur is sulfide, not sulfurde.
Worksheet Names of Ionic Compounds
The following worksheet includes a list of ionic compounds. Organize the worksheet into columns for the cation, anion, and name of the compound. Include a section for students to practice naming ionic compounds.
| Cation | Anion | Name |
|---|---|---|
| Na+ | Cl- | Sodium chloride |
| K+ | I- | Potassium iodide |
| Ca2+ | O2- | Calcium oxide |
Students can practice naming ionic compounds by completing the following exercises:
- Name the ionic compound formed between sodium and chlorine.
- Name the ionic compound formed between potassium and iodine.
- Name the ionic compound formed between calcium and oxygen.
Activities and Examples, Worksheet names of ionic compounds
| Ionic Compound | Name |
|---|---|
| NaCl | Sodium chloride |
| KI | Potassium iodide |
| CaO | Calcium oxide |
Here are some practice problems for students to name ionic compounds:
- Name the ionic compound formed between magnesium and fluorine.
- Name the ionic compound formed between aluminum and oxygen.
- Name the ionic compound formed between iron(II) and chlorine.
Ionic compounds are used in a variety of everyday applications, such as table salt (NaCl), baking soda (NaHCO3), and plaster of Paris (CaSO4).
Further Exploration
Ionic compounds have a wide range of applications in everyday life, from the food we eat to the medicines we take. They also play an important role in chemical reactions, such as the neutralization of acids and bases.
Here are some additional resources for learning about ionic compounds:
FAQ Resource
What are ionic compounds?
Ionic compounds are chemical substances formed by the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions).
How are ionic compounds named?
Ionic compounds are named based on the names of the constituent ions. The cation is named first, followed by the anion.
What are some examples of ionic compounds?
Common examples of ionic compounds include sodium chloride (NaCl), potassium chloride (KCl), and calcium oxide (CaO).