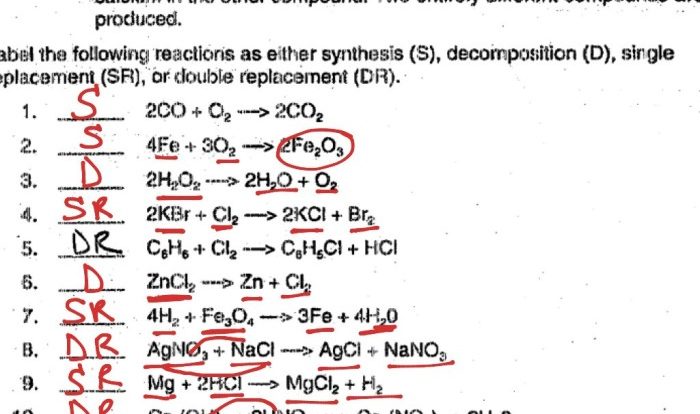
Delve into the fascinating world of chemical reactions with our comprehensive five types of chemical reactions worksheet. This in-depth guide will equip you with a thorough understanding of the fundamental types of chemical reactions, their characteristics, and their applications in various fields.
As you embark on this educational journey, you will explore the intricacies of synthesis reactions, decomposition reactions, single-displacement reactions, double-displacement reactions, and combustion reactions. Each type of reaction is meticulously defined, illustrated with real-world examples, and supported by clear explanations of reactants and products.
Introduction
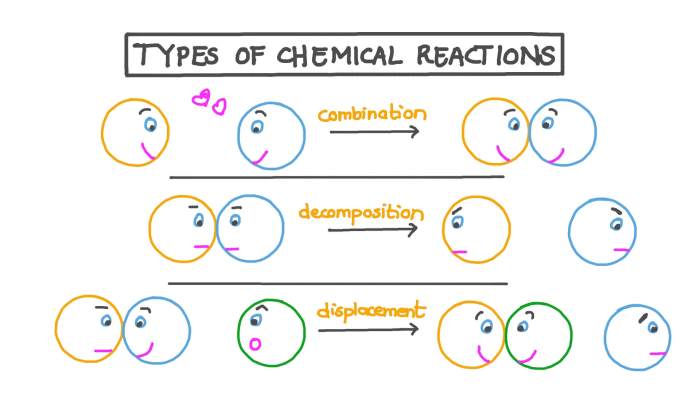
Chemical reactions are fundamental processes that drive countless transformations in our everyday lives. From the digestion of food to the combustion of fuels, chemical reactions play a crucial role in shaping our world. This worksheet provides an overview of the five main types of chemical reactions, their characteristics, and their applications.
Synthesis Reactions
Synthesis reactions involve the combination of two or more reactants to form a single, more complex product. The general formula for a synthesis reaction is:
A + B → AB
In a synthesis reaction, the reactants are the starting materials, and the product is the newly formed substance.
Decomposition Reactions, Five types of chemical reactions worksheet
Decomposition reactions are the opposite of synthesis reactions. They involve the breakdown of a single reactant into two or more simpler products. The general formula for a decomposition reaction is:
AB → A + B
In a decomposition reaction, the reactant is the starting material, and the products are the substances that are formed.
Single-Displacement Reactions
Single-displacement reactions involve the replacement of one element in a compound by another element. The general formula for a single-displacement reaction is:
A + BC → AC + B
In a single-displacement reaction, the reactant A replaces the element B in the compound BC, forming the new compound AC and releasing the element B.
Double-Displacement Reactions
Double-displacement reactions involve the exchange of ions between two compounds. The general formula for a double-displacement reaction is:
AB + CD → AD + CB
In a double-displacement reaction, the cations (positively charged ions) of the two compounds switch places, forming two new compounds.
Combustion Reactions
Combustion reactions are chemical reactions that involve the rapid reaction of a fuel with oxygen, releasing heat and light. The general formula for a combustion reaction is:
Fuel + O 2→ CO 2+ H 2O + Energy
In a combustion reaction, the fuel is the reactant, and the products are carbon dioxide, water, and energy.
Quick FAQs: Five Types Of Chemical Reactions Worksheet
What are the five main types of chemical reactions?
Synthesis, decomposition, single-displacement, double-displacement, and combustion reactions.
What is the general formula for a synthesis reaction?
A + B → AB
What is the difference between a reactant and a product in a chemical reaction?
Reactants are the initial substances that undergo a chemical change, while products are the new substances formed as a result of the reaction.