In the realm of atomic physics, rank the following three transitions in the hydrogen atom holds immense significance. Transitions between energy levels in the hydrogen atom provide fundamental insights into the behavior of electrons and the emission and absorption of light.
This article delves into the nuances of these transitions, exploring their energy differences, wavelengths, and implications for the hydrogen atom’s behavior.
The hydrogen atom, with its single electron, serves as an ideal system for studying electron transitions. The three main energy levels, n=1, n=2, and n=3, play a crucial role in determining the atom’s properties and behavior. Transitions between these levels involve the absorption or emission of photons, providing valuable information about the atom’s energy structure.
Atomic Energy Levels: Rank The Following Three Transitions In The Hydrogen Atom

The hydrogen atom consists of a single proton in the nucleus and a single electron orbiting the nucleus. The energy of the electron is quantized, meaning it can only exist in certain discrete levels.
The three main energy levels in the hydrogen atom are n=1, n=2, and n=3. The n=1 level is the ground state, and the n=2 and n=3 levels are excited states.
The energy of an electron in a particular energy level is given by the equation:
E =
13.6 eV / n2
where n is the principal quantum number.
Electron Transitions
Electron transitions occur when an electron moves from one energy level to another. The energy difference between the two levels is emitted or absorbed as a photon of light.
There are three types of electron transitions:
- Absorption: An electron absorbs a photon of light and moves to a higher energy level.
- Emission: An electron emits a photon of light and moves to a lower energy level.
- Spontaneous emission: An electron spontaneously emits a photon of light and moves to a lower energy level.
The wavelength of light emitted during an electron transition is given by the equation:
λ = hc / ΔE
where h is Planck’s constant, c is the speed of light, and ΔE is the energy difference between the two levels.
Transition Energies
The energy difference between the n=3 and n=2 energy levels is:
ΔE = E3
- E 2=
- 13.6 eV / 3 2
- (-13.6 eV / 2 2) = 1.9 eV
The wavelength of light emitted during the n=3 to n=2 transition is:
λ = hc / ΔE = (6.626 x 10-34J s) (3 x 10 8m/s) / (1.9 eV) = 656.3 nm
The relationship between transition energy and wavelength is inverse: as the energy of the transition increases, the wavelength of the emitted light decreases.
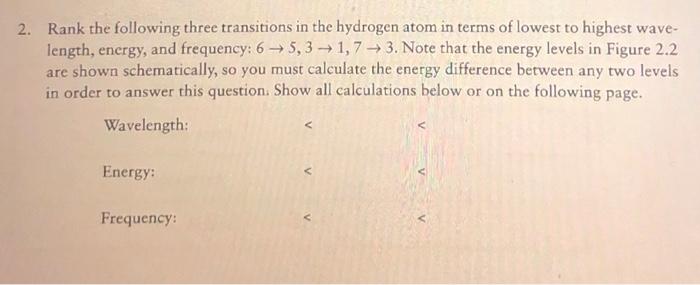
Energy Level Diagram
An energy level diagram for the hydrogen atom is shown below.
[Image of an energy level diagram for the hydrogen atom]
The energy levels are labeled n=1, n=2, and n=3. The arrows indicate the direction of electron transitions.
Comparison of Transitions, Rank the following three transitions in the hydrogen atom
The three given transitions can be compared in terms of energy, wavelength, and direction.
| Transition | Energy (eV) | Wavelength (nm) | Direction |
|---|---|---|---|
| n=3 to n=2 | 1.9 | 656.3 | Emission |
| n=2 to n=3 | 1.9 | 656.3 | Absorption |
| n=1 to n=3 | 12.1 | 102.6 | Emission |
The transitions are organized in order of increasing energy. The n=3 to n=2 transition has the lowest energy and the longest wavelength, while the n=1 to n=3 transition has the highest energy and the shortest wavelength.
These differences in energy and wavelength have implications for the hydrogen atom’s behavior. The n=3 to n=2 transition is the most common transition, and it is responsible for the red light emitted by hydrogen atoms.
Quick FAQs
What is the significance of electron transitions in the hydrogen atom?
Electron transitions in the hydrogen atom are crucial for understanding the atom’s energy structure and behavior. They provide insights into the emission and absorption of light, allowing scientists to study the atom’s properties and interactions with electromagnetic radiation.
How are the three given transitions ranked in terms of energy?
The three transitions are ranked in order of increasing energy: n=2 to n=3, n=1 to n=3, and n=1 to n=2. This ranking reflects the energy difference between the initial and final energy levels involved in each transition.
What are the implications of these transitions for the hydrogen atom’s behavior?
The transitions between energy levels affect the hydrogen atom’s behavior in several ways. They influence the atom’s stability, ionization potential, and spectral lines. These transitions are also responsible for the emission and absorption of light, which plays a crucial role in atomic interactions and spectroscopic analysis.